MEEG Automated Workflow System (MEAW SYSTEMTM®)
By Precision Medicine Centre, Hokuto Hospital, Japan

Just load your data using the GUI and perform statistical easily! MEAW SYSTEM® is a Statistical Parametric Mapping (SPM, https://www.fil.ion.ucl.ac.uk/spm/)-based toolbox which allows you to analyse Magnetoencephalography (MEG) data without any troublesome procedures or operations.
We also provide control/reference data (Hokuto 102), which is comparable with data from patients/participants.
MEAW SYSTEM® enables you to concentrate on your own study goals and patients.
To download MEAW SYSTEM® and Hokuto 102 or obtain more information,
email us: meaw.system[at]gmail.com (Please replace [at] with @)
Could you please make the title ‘Inquiry about MEAW SYSTEM®‘?
Hideyuki HOSHI, System Engineering and Researcher
Yoshihito SHIGIHARA, MD PhD, Director
MEAW developing team in Precision Medicine Centre, Hokuto Hospital, Japan
We thank Dr Samuel CHEADLE for editing English in this homepage
This study was partially supported by RICOH.
The sponsor has no control over designing the study, recoding and analysing data in this study.
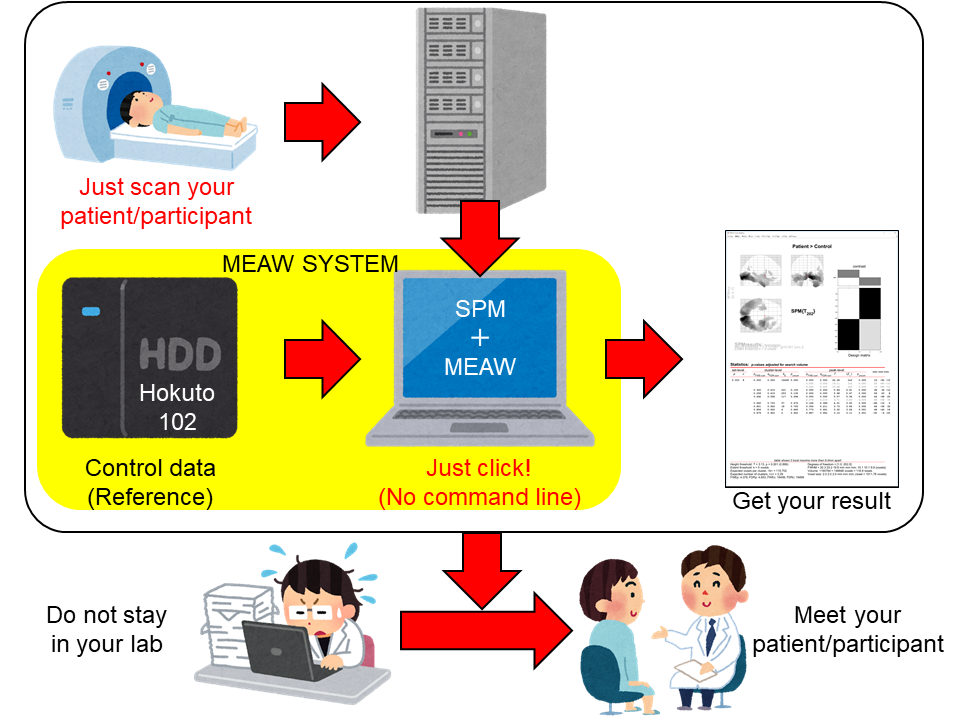
Figure 1. Concept of analysis using MEAW SYSTEM®
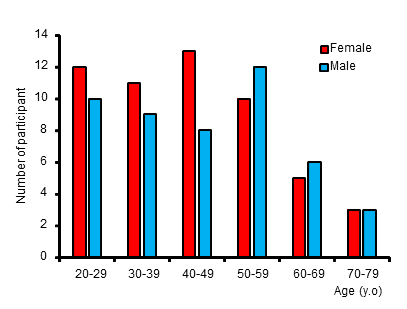
Figure 2. Histogram for age